Help Transform Recovery1‑3

Jump to

Jump to
SUBLOCADE® offers reliable buprenorphine delivery with once‑monthly dosing1
Straightforward dosing for continuous buprenorphine delivery all month long1
May align with patient preferences regarding consistent medication delivery all month, discreet administration to help with stigma, and the burden of daily dosing4-6
Support patients in focusing on comprehensive recovery support rather than negative daily drug-taking rituals and habits2,4,5,7

Straightforward, accelerated dosing1
Designed for all appropriate patients to start SUBLOCADE on Day 11
Initiate SUBLOCADE treatment at 300 mg
for 2 injections1
Initiate
DAY 1
For patients not on buprenorphine1
Establish tolerability with a dose of TM BUP (eg, 4 mg) and observe for 1 hour prior to starting SUBLOCADE on Day 1
- TM BUP should be initiated when clear signs of opioid withdrawal are evident
- Monitor patients in a healthcare setting after injection to assess for symptoms of worsening withdrawal or sedation. Patients' symptoms should be stable or improving prior to release from the healthcare setting
- On Day 1, up to an additional 8 mg of TM BUP could be administered as needed to manage withdrawal symptoms
For patients already receiving TM BUP (8-24 mg/day)1
Transition directly to SUBLOCADE on Day 1
- Patients established on long-term doses of buprenorphine (8-18 mg/day) are eligible to reduce their dose to 100 mg at the second injection if opioid withdrawal and cravings are controlled
TM BUP=transmucosal buprenorphine.
For patients not on buprenorphine1
Establish tolerability with a dose of TM BUP (eg, 4 mg) and observe for 1 hour prior to starting SUBLOCADE on Day 1
- TM BUP should be initiated when clear signs of opioid withdrawal are evident
- Monitor patients in a healthcare setting after injection to assess for symptoms of worsening withdrawal or sedation. Patients' symptoms should be stable or improving prior to release from the healthcare setting
- On Day 1, up to an additional 8 mg of TM BUP could be administered as needed to manage withdrawal symptoms
For patients already receiving TM BUP (8-24 mg/day)1
Transition directly to SUBLOCADE on Day 1
- Patients established on long-term doses of buprenorphine (8-18 mg/day) are eligible to reduce their dose to 100 mg at the second injection if opioid withdrawal and cravings are controlled
TM BUP=transmucosal buprenorphine.
Accelerate
AS EARLY AS DAY 8
(AND UP TO 1 MONTH AFTER FIRST INJECTION)
The second injection may be administered as early as 1 week and up to 1 month after the initial injection, based on patient need1
The second injection may be administered as early as 1 week and up to 1 month after the initial injection, based on patient need1
Continue SUBLOCADE monthly injections1
Activate
MONTHLY
Both doses of SUBLOCADE provide sufficient sustained therapeutic buprenorphine plasma levels all month1,3
The 300-mg dose provides higher steady-state buprenorphine plasma levels (around 5-6 ng/mL) that some patients may need depending on their clinical condition1,3
Both doses of SUBLOCADE provide sufficient sustained therapeutic buprenorphine plasma levels all month1,3
The 300-mg dose provides higher steady-state buprenorphine plasma levels (around 5-6 ng/mL) that some patients may need depending on their clinical condition1,3
Real-world, post-marketing data indicate that HCPs prescribed some patients the SUBLOCADE 300‑mg maintenance dose9
Nearly half (47.8%) of patients were prescribed at least one 300‑mg maintenance dose following 2 initial doses of 300 mg9†‡
In the Phase 3 double-blind, placebo-controlled study, there was no difference in efficacy between the 300‑mg and 100‑mg doses1,3
†Post-marketing data were analyzed for 21,224 US patients who were prescribed at least 4 SUBLOCADE injections from April 1, 2023 to September 30, 2024. Includes patients who were prescribed 300 mg for their third and fourth injection (40%) and patients whose dose was increased back to 300 mg following a dose reduction at their third or fourth injection to 100 mg (8%).9
‡Based on dispensed units and does not necessarily indicate the medication was given.9
To achieve
steady state
Monthly
Steady state was achieved after 4-6 monthly injections of SUBLOCADE1
Steady state was achieved after 4-6 monthly injections of SUBLOCADE1
Real-world, post-marketing data indicate that HCPs prescribed some patients the SUBLOCADE 300‑mg maintenance dose9
Nearly half (47.8%) of patients were prescribed at least one 300‑mg maintenance dose following 2 initial doses of 300 mg9†‡
In the Phase 3 double-blind, placebo-controlled study, there was no difference in efficacy between the 300‑mg and 100‑mg doses1,3
†Post-marketing data were analyzed for 21,224 US patients who were prescribed at least 4 SUBLOCADE injections from April 1, 2023 to September 30, 2024. Includes patients who were prescribed 300 mg for their third and fourth injection (40%) and patients whose dose was increased back to 300 mg following a dose reduction at their third or fourth injection to 100 mg (8%).9
‡Based on dispensed units and does not necessarily indicate the medication was given.9
- Administer SUBLOCADE maintenance doses monthly with at least 26 days between doses1
- Strongly consider prescribing naloxone along with SUBLOCADE because patients being treated for OUD are at risk of overdose1
Real-world, post-marketing data indicate that HCPs prescribed some patients the SUBLOCADE 300‑mg maintenance dose9
Nearly half (47.8%) of patients were prescribed at least one 300‑mg maintenance dose following 2 initial doses of 300 mg9†‡
In the Phase 3 double-blind, placebo-controlled study, there was no difference in efficacy between the 300‑mg and 100‑mg doses1,3
†Post-marketing data were analyzed for 21,224 US patients who were prescribed at least 4 SUBLOCADE injections from April 1, 2023 to September 30, 2024. Includes patients who were prescribed 300 mg for their third and fourth injection (40%) and patients whose dose was increased back to 300 mg following a dose reduction at their third or fourth injection to 100 mg (8%).9
‡Based on dispensed units and does not necessarily indicate the medication was given.9
- If a patient misses a maintenance dose, administer the next dose as soon as possible, with the following dose given at least 26 days later1
- Occasional delays in dosing up to 2 weeks are not expected to have a clinically significant impact on treatment effect1
OUD=opioid use disorder.


YOUR PATIENTS' PREFERENCES MATTER2*
From day 1: 4 injection sites to choose from1
SUBLOCADE offers injection site options with continuous buprenorphine delivery all month1
4 subcutaneous injection site options1
- Abdomen
- Buttock
- Thigh
- Back of the upper arm
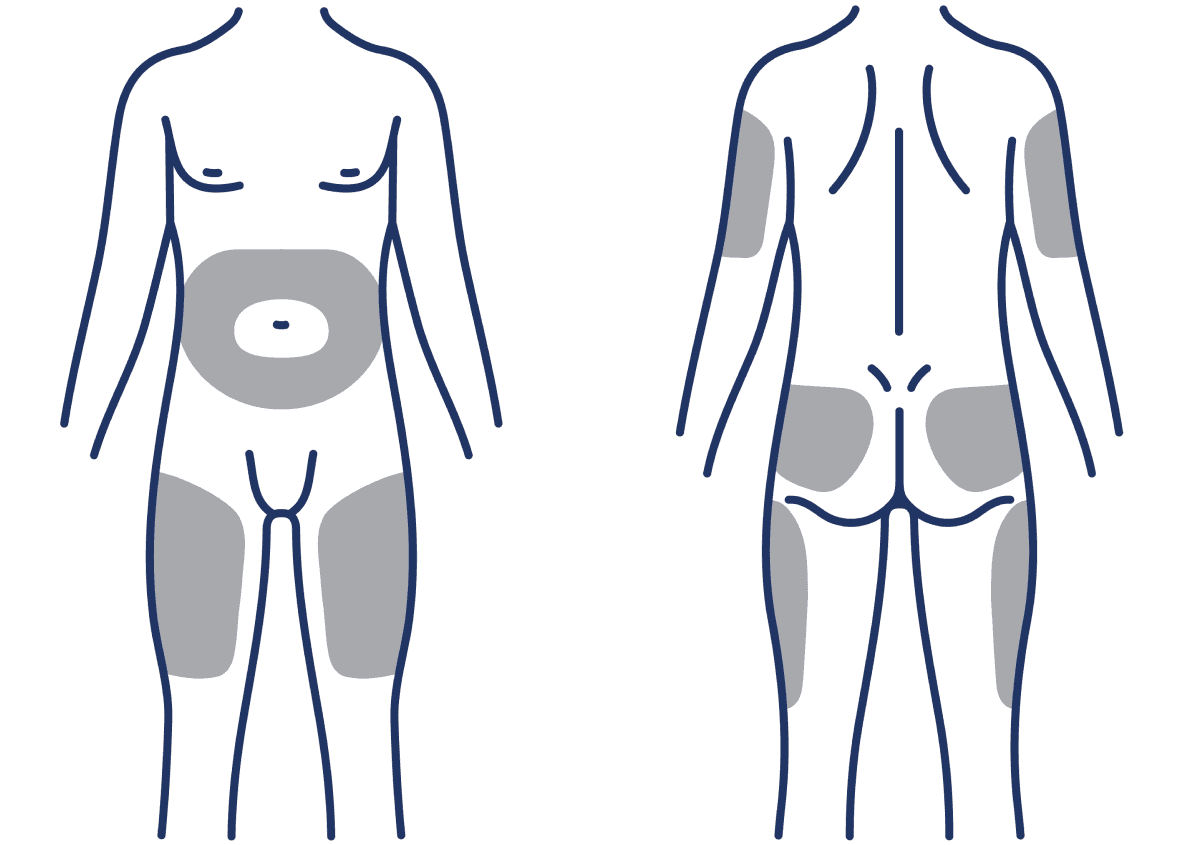
No dose adjustments based on injection site location are needed. To avoid irritation, rotate injection sites.1
In a Phase 4, single-dose study of alternative subcutaneous injection sites,† there were no estimated differences in the incidence of injection site reaction AEs with SUBLOCADE administered in back of upper arm, thigh, or buttock vs the abdomen.9, 10‡

*Patient preference was not evaluated in this study. All participants were randomized to 1 of the 4 treatment arms (injection locations).
†Study evaluated the relative bioavailability (primary objective), safety, and tolerability of the alternative sites compared with the abdomen.10
‡Safety results were summarized using descriptive statistics and any differences between alternative injection locations vs the abdomen were estimated with a 95% CI.10
AE=adverse event; CI=confidence interval.

Administering SUBLOCADE
SUBLOCADE administration offers choices of injection site with continuous buprenorphine delivery all month1
- SUBLOCADE is for subcutaneous injection only; do not administer SUBLOCADE intravenously, intramuscularly, or intradermally. Serious harm or death could result if administered intravenously1
- SUBLOCADE is to be prepared and administered by a healthcare provider only1*
- For patients not previously on buprenorphine, monitor in a healthcare setting after injection to assess for symptoms of worsening withdrawal or sedation until symptoms are stable or improving1
*Includes physicians, nurse practitioners, physician's assistants, or suitable qualified designees (eg, nursing staff) as authorized to administer controlled substances under federal, state, or local laws and regulations.
Order SUBLOCADE 300‑mg syringe
Dispense 1 syringe*
Administer the first syringe/dose on Day 1
Refills: 1, available as early as day 8 and up to 26 days† after initial dispense per HCP direction
*SUBLOCADE should only be dispensed directly to a healthcare provider for administration by a healthcare provider.
†The order for the refill should be submitted by Day 2 to ensure on-time delivery from the pharmacy. The second dose may be administered as early as 1 week and up to 1 month after the initial injection, based on patient need. Subsequent doses should be administered with at least 26 days between injections.
SUBLOCADE CAN REMAIN AT ROOM TEMPERATURE FOR UP TO 12 WEEKS
Allows for storage flexibility and possibly less product waste for your practice
SUBLOCADE is available in a single-dose, prefilled syringe with safety needle.
- Store refrigerated at 35.6°F‑46.4°F (2°C‑8°C)
- Remove SUBLOCADE from refrigerator at least 15 minutes prior to administration to allow it to come to room temperature (59°F to 86°F)
- Do not open the foil pouch until the patient has arrived for their injection
- Once outside the refrigerator, SUBLOCADE may be stored in its original packaging at room temperature for up to 12 weeks prior to administration. Discard if left at room temperature for more than 12 weeks
- SUBLOCADE is a Schedule III drug product. Handle with adequate security and accountability
Refer to the full Prescribing Information inside the product packaging to ensure proper storage and safe usage.
- Patients can stop taking SUBLOCADE at any time, if desired or necessary
- If considering stopping treatment, the clinical status of the patient should be considered
- Patients who elect to discontinue treatment with SUBLOCADE should be monitored for several months for withdrawal signs and symptoms
- Remove the depot (if necessary)
- In the event the depot must be removed, it can be surgically excised by an HCP under local anesthesia within 14 days of injection. Only the most recently injected depot can be removed. Patients who have the depot removed should be monitored for signs and symptoms of withdrawal and treated appropriately
- Only 2 cases of surgical removal of the depot were reported in clinical studies
- Tapering was not studied in clinical trials for the discontinuation of SUBLOCADE
- Plasma levels decrease slowly
- Model simulations indicate that steady-state buprenorphine plasma concentrations decreased slowly over time following the last injection. Concentration levels remained at therapeutic levels for 2 to 5 months on average, depending on the dosage administered (100 or 300 mg, respectively)
- After achieving steady state, patients discontinuing SUBLOCADE may have detectable plasma and urine levels of buprenorphine for 12 months or longer
- Withdrawal of a partial agonist is typically milder than that of a full agonist and may be delayed in onset
- Due to the long‑acting nature of SUBLOCADE, withdrawal signs and symptoms may not be evident immediately following discontinuation. Patients should be monitored for signs and symptoms of withdrawal
- Consider TM BUP, if needed, to treat withdrawal following discontinuation of SUBLOCADE
INDICATION
SUBLOCADE, with counseling and psychosocial support, is for moderate to severe opioid use disorder in those who have initiated treatment with a dose of transmucosal buprenorphine or are being treated with buprenorphine.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF SERIOUS HARM OR DEATH WITH INTRAVENOUS ADMINISTRATION; SUBLOCADE RISK EVALUATION AND MITIGATION STRATEGY
- Serious harm or death could result if administered intravenously. SUBLOCADE forms a solid mass upon contact with body fluids and may cause occlusion, local tissue damage, and thrombo-embolic events, including life threatening pulmonary emboli, if administered intravenously.
- Because of the risk of serious harm or death that could result from intravenous self-administration, SUBLOCADE is only available through a restricted program called the SUBLOCADE REMS Program. Healthcare settings and pharmacies that order and dispense SUBLOCADE must be certified in this program and comply with the REMS requirements.
CONTRAINDICATIONS: SUBLOCADE should not be administered to patients who are hypersensitive to buprenorphine or any component of the delivery system.
WARNINGS AND PRECAUTIONS
Addiction, Abuse, and Misuse: SUBLOCADE contains buprenorphine, a Schedule III controlled substance that can be abused in a manner similar to other opioids. Buprenorphine is sought by people with opioid use disorder and is subject to criminal diversion. Monitor patients for conditions indicative of diversion or progression of opioid dependence and addictive behaviors.
Risk of Life-Threatening Respiratory Depression and Concomitant Use of Benzodiazepines or Other CNS Depressants with Buprenorphine: Buprenorphine has been associated with life-threatening respiratory depression, overdose, and death, particularly when misused by self-injection or with concomitant use of benzodiazepines or other CNS depressants, including alcohol. Warn patients of the potential danger of self-administration of benzodiazepines, other CNS depressants, opioid analgesics, and alcohol while under treatment with SUBLOCADE. Counsel patients that such medications should not be used concomitantly unless supervised by a healthcare provider.
Use with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression).
Opioids can cause sleep-related breathing disorders, e.g., central sleep apnea (CSA), sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. Consider decreasing the opioid using best practices for opioid taper if CSA occurs.
Strongly consider prescribing naloxone at the time SUBLOCADE is initiated or renewed because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose. Educate patients and caregivers on how to recognize respiratory depression and, if naloxone is prescribed, how to treat with naloxone. Emphasize the importance of calling 911 or getting emergency help, even if naloxone is administered.
Risk of Serious Injection Site Reactions: The most common injection site reactions are pain, erythema and pruritus with some involving abscess, ulceration, and necrosis. Some cases resulted in surgical depot removal, debridement, antibiotic administration, and SUBLOCADE discontinuation. The likelihood of serious injection site reactions may increase with inadvertent intramuscular or intradermal administration. Carefully review injection technique.
Neonatal Opioid Withdrawal Syndrome: Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy. NOWS may be life-threatening if not recognized and treated in the neonate. Newborns should be observed for signs of NOWS and managed accordingly. Advise pregnant women receiving opioid addiction treatment with SUBLOCADE of the risk of neonatal opioid withdrawal syndrome.
Adrenal Insufficiency: Adrenal insufficiency has been reported with opioid use. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off the opioid.
Discontinuation of SUBLOCADE Treatment: Due to the long-acting nature of SUBLOCADE, if treatment is discontinued, monitor patients for several months for withdrawal and treat appropriately.
Inform patients that they may have detectable levels of buprenorphine for a prolonged period of time after treatment with SUBLOCADE. Considerations of drug-drug interactions, buprenorphine effects, and analgesia may continue to be relevant for several months after the last injection.
Risk of Hepatitis, Hepatic Events: Because cases of cytolytic hepatitis and hepatitis with jaundice have been observed in individuals receiving buprenorphine, monitor liver function tests prior to treatment and monthly during treatment.
Hypersensitivity Reactions: Hypersensitivity to buprenorphine-containing products have been reported most commonly as rashes, hives, and pruritus. Some cases of bronchospasm, angioneurotic edema, and anaphylactic shock have also been reported.
Precipitation of Opioid Withdrawal in Patients Dependent on Full Agonist Opioids: Buprenorphine may precipitate opioid withdrawal signs and symptoms in persons who are currently physically dependent on full opioid agonists if administered before the effects have subsided, at least 6 hours for short-acting opioids and 24 hours for long-acting opioids. Verify that patients have tolerated transmucosal buprenorphine before administering the first injection of SUBLOCADE.
Risks Associated With Treatment of Emergent Acute Pain: When patients need acute pain management, or may require anesthesia, treat patients receiving SUBLOCADE currently or within the last 6 months with a non-opioid analgesic whenever possible. If opioid therapy is required, patients may be treated with a high-affinity full opioid analgesic under the supervision of a physician, with particular attention to respiratory function, as higher doses may be required for analgesic effect and therefore, a higher potential for toxicity exists with opioid administration.
Advise patients of the importance of instructing their family members, in the event of emergency, to inform the treating healthcare provider or emergency room staff that the patient is physically dependent on an opioid and that the patient is being treated with SUBLOCADE.
Use in Opioid Naïve Patients: Because death has been reported for opioid naïve individuals who received buprenorphine sublingual tablet, SUBLOCADE is not appropriate for use in opioid naïve patients.
Use in Patients With Impaired Hepatic Function: Because buprenorphine levels cannot be rapidly decreased, SUBLOCADE is not recommended for patients with pre-existing moderate to severe hepatic impairment. Patients who develop moderate to severe hepatic impairment while being treated with SUBLOCADE should be monitored for several months for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine.
QTc Prolongation: QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. Buprenorphine is unlikely to be pro-arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT-prolonging agents is not known. Consider these observations when prescribing SUBLOCADE to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long-QT syndrome, or severe hypomagnesemia.
Impairment of Ability to Drive or Operate Machinery: SUBLOCADE may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery. Caution patients about driving or operating hazardous machinery until they are reasonably certain that SUBLOCADE does not adversely affect their ability to engage in such activities.
Orthostatic Hypotension: Buprenorphine may produce orthostatic hypotension.
Elevation of Cerebrospinal Fluid Pressure: Buprenorphine may elevate cerebrospinal fluid pressure and should be used with caution in patients with head injury, intracranial lesions, and other circumstances when cerebrospinal pressure may be increased. Buprenorphine can produce miosis and changes in the level of consciousness that may interfere with patient evaluation.
Elevation of Intracholedochal Pressure: Buprenorphine has been shown to increase intracholedochal pressure, as do other opioids, and thus should be administered with caution to patients with dysfunction of the biliary tract.
Effects in Acute Abdominal Conditions: Buprenorphine may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Unintentional Pediatric Exposure: Buprenorphine can cause severe, possibly fatal, respiratory depression in children who are accidentally exposed to it.
ADVERSE REACTIONS: Adverse reactions commonly associated with SUBLOCADE (≥5% of subjects) during clinical trials were constipation, headache, nausea, vomiting, increased hepatic enzymes, fatigue, and injection site pain and pruritus. This is not a complete list of potential adverse events. Please see the full Prescribing Information for a complete list.
DRUG INTERACTIONS
CYP3A4 Inhibitors and Inducers: Monitor patients starting or ending CYP3A4 inhibitors or inducers for potential over- or under-dosing.
Serotonergic Drugs: If concomitant use with serotonergic drugs is warranted, monitor for serotonin syndrome, particularly during treatment initiation, and during dose adjustment of the serotonergic drug.
Consult the full Prescribing Information for SUBLOCADE for more information on potentially significant drug interactions.
USE IN SPECIFIC POPULATIONS
Pregnancy: Opioid-dependent women on buprenorphine maintenance therapy may require additional analgesia during labor.
Lactation: Buprenorphine passes into the mother's milk. Advise breastfeeding women to monitor the infant for increased drowsiness and breathing difficulties.
Fertility: Chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible.
Geriatric Patients: Monitor geriatric patients receiving SUBLOCADE for sedation or respiratory depression.
To report a pregnancy or side effects associated with taking SUBLOCADE or any safety related information, product complaint, request for medical information, or product query, please contact PatientSafetyNA@indivior.com or 1-877-782-6966.
See full Prescribing Information, including Boxed Warning, and Medication Guide. For REMS information visit www.sublocadeREMS.com.
P-BAG-US-01763 May 2025


