Help Transform Recovery1‑3

Jump to

Jump to
SUBLOCADE® delivers continuous and long-lasting plasma levels all month1,4-6
On Day 1,* SUBLOCADE helped patients achieve buprenorphine plasma levels of 2+ ng/mL6
- With both rapid initiationon Day 1 andThe first 300-mg SUBLOCADE injection given on Day 1 after a dose of 4 mg TM BUP, for tolerability. 88% of patients did not require additional TM BUP on Day 11,6standard induction, average buprenorphine plasma levels were >2 ng/mL at first measurement (2 hours post-injection)6The first 300-mg SUBLOCADE injection, given following ≥7 days of TM BUP1
- Before the second injection, given as early as Day 8 in most patients, average plasma levels were 1.78 and 1.76 ng/mL in the rapid initiation and standard induction arms, respectively6
*First SUBLOCADE injection given after a dose of TM BUP (4 mg), for tolerability.1
†First SUBLOCADE injection given after ≥7 days of TM BUP.1

- With both rapid initiationon Day 1 andThe first 300-mg SUBLOCADE injection given on Day 1 after a dose of 4 mg TM BUP, for tolerability. 88% of patients did not require additional TM BUP on Day 11,6standard induction, average buprenorphine plasma levels were >2 ng/mL at first measurement (2 hours post-injection)6The first 300-mg SUBLOCADE injection, given following ≥7 days of TM BUP1
- Before the second injection, given as early as Day 8 in most patients, average plasma levels were 1.78 and 1.76 ng/mL in the rapid initiation and standard induction arms, respectively6
*First SUBLOCADE injection given after a dose of TM BUP (4 mg), for tolerability.1
†First SUBLOCADE injection given after ≥7 days of TM BUP.1
(INDV-6000-401, NCT04995029)1
High-risk opioid use was defined as using at least one of the following: 5 or more days per week via injection, high doses (≥500-mg IV heroin equivalent), or potent synthetic opioids (eg, fentanyl)1
IV=intravenous.
- Rapid initiation: first 300‑mg injection given on Day 1 after a dose of 4 mg TM BUP. Participants were observed for at least 1 hour to confirm tolerability prior to the first 300‑mg SUBLOCADE injection
- On Day 1, up to an additional 8 mg of TM BUP could be administered to manage withdrawal symptoms
- Standard induction: first 300‑mg injection given after a minimum of 7 days of TM BUP
In both treatment groups, the second 300‑mg injection was administered as early as 1 week after the first SUBLOCADE injection and subsequent injections were scheduled every 4 weeks.1
Primary endpoint: Participant retention at the second injection of SUBLOCADE. Data is based on 723 participants who were able to be evaluated for the primary endpoint.6*
Exploratory endpoint: Buprenorphine plasma levels measured after subcutaneous injections of SUBLOCADE.6
- Data included all participants who had at least one buprenorphine plasma concentration measurement (N=397 rapid initiation; N=151 standard induction)
- Plasma levels were measured on Day 1 pre-injection and 2 hours post-injection as well as later time points (Days 8, 15, 22, 29, and 36)
*Excludes participants who discontinued treatment due to not meeting inclusion/exclusion criteria.6
OUD=opioid use disorder; TM BUP=transmucosal buprenorphine.

Administering the second 300-mg SUBLOCADE dose on Day 8 may help reach and maintain plasma levels of 2+ ng/mL earlier than giving it at Day 296
Data based on PK simulation.6
Plasma concentrations were simulated for the 300 mg/100 mg dosing regimen following 1 week of TM BUP (16 mg/day), with the second dose of SUBLOCADE received at 1 week (Day 8) or 4 weeks (Day 29) after the first dose.6
Pharmacokinetic simulations were conducted using the referenced population pharmacokinetic model for SUBLOCADE.6
- Simulated concentrations were summarized by the median (solid lines) and 90% prediction interval (shaded areas) at each time point
- Simulated data depicted through Week 16 after the first dose (17 weeks total) and 4 SUBLOCADE doses (two 300‑mg doses, followed by two 100‑mg doses)
- Observed plasma levels measured after Injection 2 from patients in the rapid induction study (data not shown) were consistent with the simulated plasma levels*
*In the rapid induction substudy, the second SUBLOCADE injection was typically given 7 days after the first injection, but dosing time varied between 4 to 28 days; 1 participant received the second injection 67 days after the first injection. Observed data from the study was reported through Day 36 (two 300‑mg SUBLOCADE doses).6
Pharmacokinetic simulations were conducted using the referenced population pharmacokinetic model for SUBLOCADE.6
- Simulated concentrations were summarized by the median (solid lines) and 90% prediction interval (shaded areas) at each time point
- Simulated data depicted through Week 16 after the first dose (17 weeks total) and 4 SUBLOCADE doses (two 300‑mg doses, followed by two 100‑mg doses)
- Observed plasma levels measured after Injection 2 from patients in the rapid induction study (data not shown) were consistent with the simulated plasma levels*
*In the rapid induction substudy, the second SUBLOCADE injection was typically given 7 days after the first injection, but dosing time varied between 4 to 28 days; 1 participant received the second injection 67 days after the first injection. Observed data from the study was reported through Day 36 (two 300‑mg SUBLOCADE doses).6
PK=pharmacokinetic; TM BUP=transmucosal buprenorphine.

A peak occurred around 24 hours, the first measurement post injection, then slowly decreased to a plateau around 2 ng/mL for the first injection and 3 ng/mL for the second injection1,3
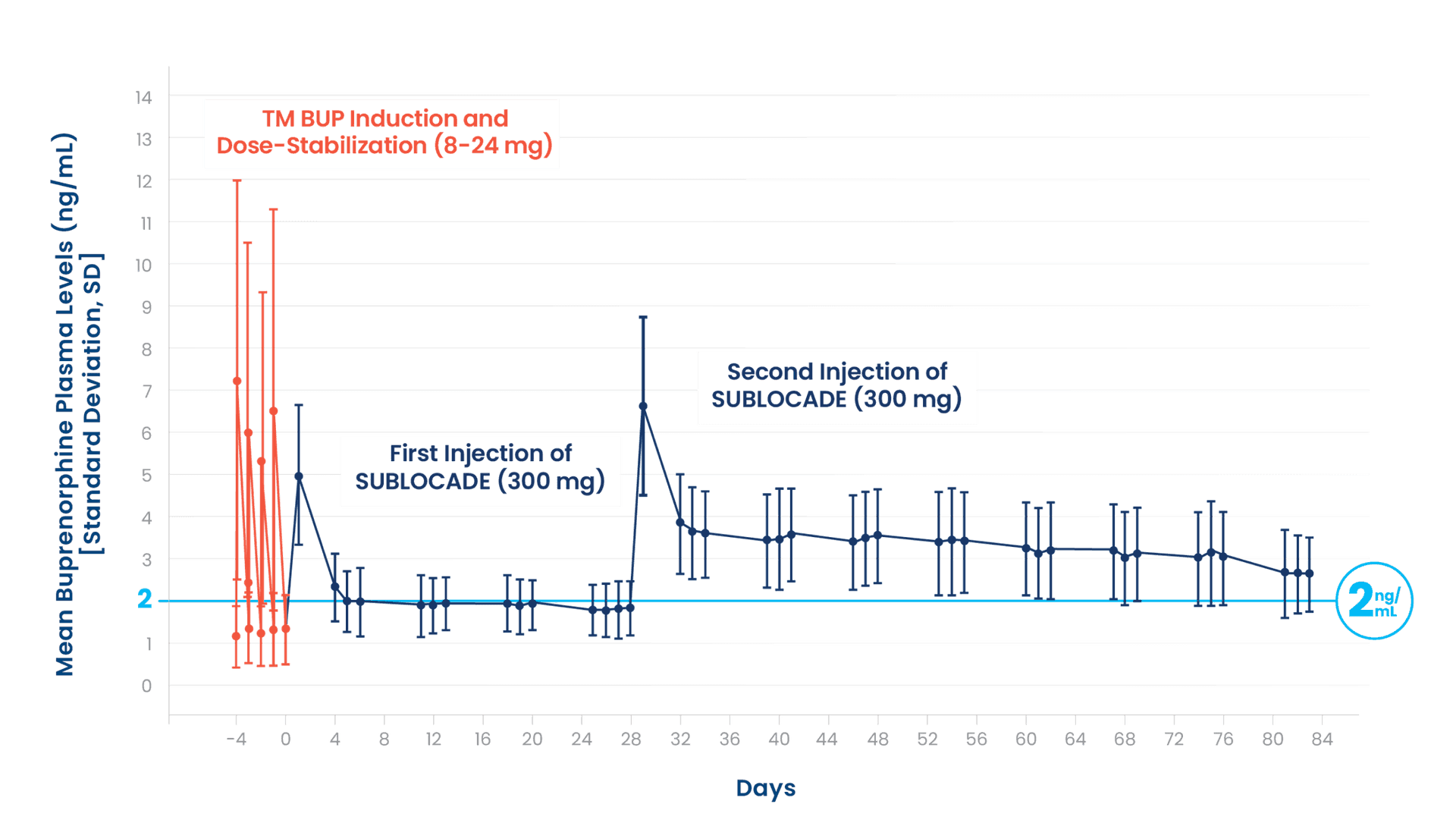
TM BUP=transmucosal buprenorphine.
(13-0002, NCT02044094)1
In this 12-week study, non‑treatment-seeking participants with OUD received 300 mg of SUBLOCADE on Day 1 (n=39) and Day 29 (n=30). Participants were challenged with both doses of hydromorphone (6‑mg and 18‑mg) or placebo (1) at baseline, (2) at the end of the induction/stabilization period of TM BUP, and (3) during each of the 12 weeks of the study for 3 consecutive days (each week on Days 5‑7) in a randomized fashion.1,6
- Prior to the first injection of SUBLOCADE, participants started with a required induction and dose-stabilization period with TM BUP. Induction occurred over 3 days and dose-stabilization over a period of 4 to 11 days. Though not seeking treatment, 77% (30/39) of participants returned for the second dose of SUBLOCADE
The study was not designed or intended to draw any head-to-head comparisons between TM BUP and SUBLOCADE.6
Primary objective: Demonstrate that the peak (Emax) effect of drug-liking for 6 mg and 18 mg intramuscular hydromorphone challenges following subcutaneous injections of SUBLOCADE 300 mg was noninferior (ie, hydromorphone was not substantially more likable) to the peak effect of drug-liking of the placebo challenge as measured by a unipolar 100‑point VAS.1,6
PK assessments: Buprenorphine plasma levels were taken (1) immediately before each hydromorphone challenge, (2) before and 1.5 hours after TM BUP, and (3) before and 24 hours after each SUBLOCADE injection.6,7
Select Inclusion criteria for participants8
Participants needed to meet DSM‑5 criteria for moderate or severe OUD at screening and not be seeking treatment for OUD
- Aged 18-55 years
- BMI 18-33.0 kg/m2
- Current or historical experience with parenteral abuse of opioids
- Experiencing signs and symptoms of withdrawal prior to the start of buprenorphine‑naloxone sublingual film dosing (as evidenced by a COWS score >12)
- Women of childbearing potential (defined as all women who are not surgically sterile or postmenopausal for ≥1 year prior to informed consent) must have a negative pregnancy test prior to enrollment and must agree to use a medically acceptable means of contraception from screening through ≥3 months after the last dose of study drug
- Male subjects with female partners of childbearing potential must agree to use medically acceptable contraception from screening through ≥3 months after the last dose of study drug
- Normal or no clinically significant ECG findings at screening
- Total bilirubin ≤1.5 × ULN, ALT ≤3 × ULN, AST ≤3 × ULN, serum creatinine ≤2 × ULN, INR ≤1.5 × ULN
ALT=alanine transaminase; AST=aspartate aminotransferase; BMI=body mass index; COWS=clinical opiate withdrawal scale; DSM‑5=Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ECG=electrocardiogram; INR=international normalized ratio; OUD=opioid use disorder; PK=pharmacokinetic; TM BUP=transmucosal buprenorphine; ULN=upper limit of normal; VAS=visual analog scale.
INDICATION
SUBLOCADE, with counseling and psychosocial support, is for moderate to severe opioid use disorder in those who have initiated treatment with a dose of transmucosal buprenorphine or are being treated with buprenorphine.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF SERIOUS HARM OR DEATH WITH INTRAVENOUS ADMINISTRATION; SUBLOCADE RISK EVALUATION AND MITIGATION STRATEGY
- Serious harm or death could result if administered intravenously. SUBLOCADE forms a solid mass upon contact with body fluids and may cause occlusion, local tissue damage, and thrombo-embolic events, including life threatening pulmonary emboli, if administered intravenously.
- Because of the risk of serious harm or death that could result from intravenous self-administration, SUBLOCADE is only available through a restricted program called the SUBLOCADE REMS Program. Healthcare settings and pharmacies that order and dispense SUBLOCADE must be certified in this program and comply with the REMS requirements.
CONTRAINDICATIONS: SUBLOCADE should not be administered to patients who are hypersensitive to buprenorphine or any component of the delivery system.
WARNINGS AND PRECAUTIONS
Addiction, Abuse, and Misuse: SUBLOCADE contains buprenorphine, a Schedule III controlled substance that can be abused in a manner similar to other opioids. Buprenorphine is sought by people with opioid use disorder and is subject to criminal diversion. Monitor patients for conditions indicative of diversion or progression of opioid dependence and addictive behaviors.
Risk of Life-Threatening Respiratory Depression and Concomitant Use of Benzodiazepines or Other CNS Depressants with Buprenorphine: Buprenorphine has been associated with life-threatening respiratory depression, overdose, and death, particularly when misused by self-injection or with concomitant use of benzodiazepines or other CNS depressants, including alcohol. Warn patients of the potential danger of self-administration of benzodiazepines, other CNS depressants, opioid analgesics, and alcohol while under treatment with SUBLOCADE. Counsel patients that such medications should not be used concomitantly unless supervised by a healthcare provider.
Use with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression).
Opioids can cause sleep-related breathing disorders, e.g., central sleep apnea (CSA), sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. Consider decreasing the opioid using best practices for opioid taper if CSA occurs.
Strongly consider prescribing naloxone at the time SUBLOCADE is initiated or renewed because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose. Educate patients and caregivers on how to recognize respiratory depression and, if naloxone is prescribed, how to treat with naloxone. Emphasize the importance of calling 911 or getting emergency help, even if naloxone is administered.
Risk of Serious Injection Site Reactions: The most common injection site reactions are pain, erythema and pruritus with some involving abscess, ulceration, and necrosis. Some cases resulted in surgical depot removal, debridement, antibiotic administration, and SUBLOCADE discontinuation. The likelihood of serious injection site reactions may increase with inadvertent intramuscular or intradermal administration. Carefully review injection technique.
Neonatal Opioid Withdrawal Syndrome: Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy. NOWS may be life-threatening if not recognized and treated in the neonate. Newborns should be observed for signs of NOWS and managed accordingly. Advise pregnant women receiving opioid addiction treatment with SUBLOCADE of the risk of neonatal opioid withdrawal syndrome.
Adrenal Insufficiency: Adrenal insufficiency has been reported with opioid use. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off the opioid.
Discontinuation of SUBLOCADE Treatment: Due to the long-acting nature of SUBLOCADE, if treatment is discontinued, monitor patients for several months for withdrawal and treat appropriately.
Inform patients that they may have detectable levels of buprenorphine for a prolonged period of time after treatment with SUBLOCADE. Considerations of drug-drug interactions, buprenorphine effects, and analgesia may continue to be relevant for several months after the last injection.
Risk of Hepatitis, Hepatic Events: Because cases of cytolytic hepatitis and hepatitis with jaundice have been observed in individuals receiving buprenorphine, monitor liver function tests prior to treatment and monthly during treatment.
Hypersensitivity Reactions: Hypersensitivity to buprenorphine-containing products have been reported most commonly as rashes, hives, and pruritus. Some cases of bronchospasm, angioneurotic edema, and anaphylactic shock have also been reported.
Precipitation of Opioid Withdrawal in Patients Dependent on Full Agonist Opioids: Buprenorphine may precipitate opioid withdrawal signs and symptoms in persons who are currently physically dependent on full opioid agonists if administered before the effects have subsided, at least 6 hours for short-acting opioids and 24 hours for long-acting opioids. Verify that patients have tolerated transmucosal buprenorphine before administering the first injection of SUBLOCADE.
Risks Associated With Treatment of Emergent Acute Pain: When patients need acute pain management, or may require anesthesia, treat patients receiving SUBLOCADE currently or within the last 6 months with a non-opioid analgesic whenever possible. If opioid therapy is required, patients may be treated with a high-affinity full opioid analgesic under the supervision of a physician, with particular attention to respiratory function, as higher doses may be required for analgesic effect and therefore, a higher potential for toxicity exists with opioid administration.
Advise patients of the importance of instructing their family members, in the event of emergency, to inform the treating healthcare provider or emergency room staff that the patient is physically dependent on an opioid and that the patient is being treated with SUBLOCADE.
Use in Opioid Naïve Patients: Because death has been reported for opioid naïve individuals who received buprenorphine sublingual tablet, SUBLOCADE is not appropriate for use in opioid naïve patients.
Use in Patients With Impaired Hepatic Function: Because buprenorphine levels cannot be rapidly decreased, SUBLOCADE is not recommended for patients with pre-existing moderate to severe hepatic impairment. Patients who develop moderate to severe hepatic impairment while being treated with SUBLOCADE should be monitored for several months for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine.
QTc Prolongation: QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. Buprenorphine is unlikely to be pro-arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT-prolonging agents is not known. Consider these observations when prescribing SUBLOCADE to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long-QT syndrome, or severe hypomagnesemia.
Impairment of Ability to Drive or Operate Machinery: SUBLOCADE may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery. Caution patients about driving or operating hazardous machinery until they are reasonably certain that SUBLOCADE does not adversely affect their ability to engage in such activities.
Orthostatic Hypotension: Buprenorphine may produce orthostatic hypotension.
Elevation of Cerebrospinal Fluid Pressure: Buprenorphine may elevate cerebrospinal fluid pressure and should be used with caution in patients with head injury, intracranial lesions, and other circumstances when cerebrospinal pressure may be increased. Buprenorphine can produce miosis and changes in the level of consciousness that may interfere with patient evaluation.
Elevation of Intracholedochal Pressure: Buprenorphine has been shown to increase intracholedochal pressure, as do other opioids, and thus should be administered with caution to patients with dysfunction of the biliary tract.
Effects in Acute Abdominal Conditions: Buprenorphine may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Unintentional Pediatric Exposure: Buprenorphine can cause severe, possibly fatal, respiratory depression in children who are accidentally exposed to it.
ADVERSE REACTIONS: Adverse reactions commonly associated with SUBLOCADE (≥5% of subjects) during clinical trials were constipation, headache, nausea, vomiting, increased hepatic enzymes, fatigue, and injection site pain and pruritus. This is not a complete list of potential adverse events. Please see the full Prescribing Information for a complete list.
DRUG INTERACTIONS
CYP3A4 Inhibitors and Inducers: Monitor patients starting or ending CYP3A4 inhibitors or inducers for potential over- or under-dosing.
Serotonergic Drugs: If concomitant use with serotonergic drugs is warranted, monitor for serotonin syndrome, particularly during treatment initiation, and during dose adjustment of the serotonergic drug.
Consult the full Prescribing Information for SUBLOCADE for more information on potentially significant drug interactions.
USE IN SPECIFIC POPULATIONS
Pregnancy: Opioid-dependent women on buprenorphine maintenance therapy may require additional analgesia during labor.
Lactation: Buprenorphine passes into the mother's milk. Advise breastfeeding women to monitor the infant for increased drowsiness and breathing difficulties.
Fertility: Chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible.
Geriatric Patients: Monitor geriatric patients receiving SUBLOCADE for sedation or respiratory depression.
To report a pregnancy or side effects associated with taking SUBLOCADE or any safety related information, product complaint, request for medical information, or product query, please contact PatientSafetyNA@indivior.com or 1-877-782-6966.
See full Prescribing Information, including Boxed Warning, and Medication Guide. For REMS information visit www.sublocadeREMS.com.
P-BAG-US-01757v2 Oct 2025